TAMPERLOKS
Limitations
- The TamperLoks Drug Testing Device provides only a qualitative, preliminary, analytical result. A secondary analytical method must be used to obtain a confirmed result. Gas chromatography/mass spectrometry (GC/MS) is the preferred confirmatory method.
- It is possible that technical or procedural errors, as well as other interfering substances in the urine specimen, may cause erroneous results.
- Adulterants such as bleach and/or alum in urine specimens may produce erroneous results regardless of the analytical method used. If adulteration is suspected, the test should be repeated with another urine specimen.
- A positive result indicates presence of the drug or its metabolites but does not indicate level of intoxication, administration route or concentration in urine.
- A negative result may not necessarily indicate drug-free urine. Negative results can be obtained when drug is present but below the cut-off level.



Interpretation of results
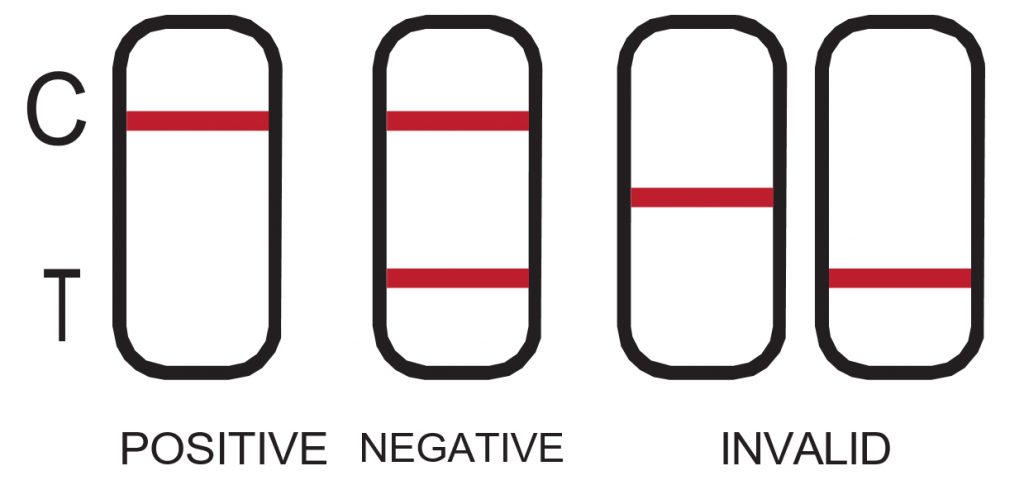

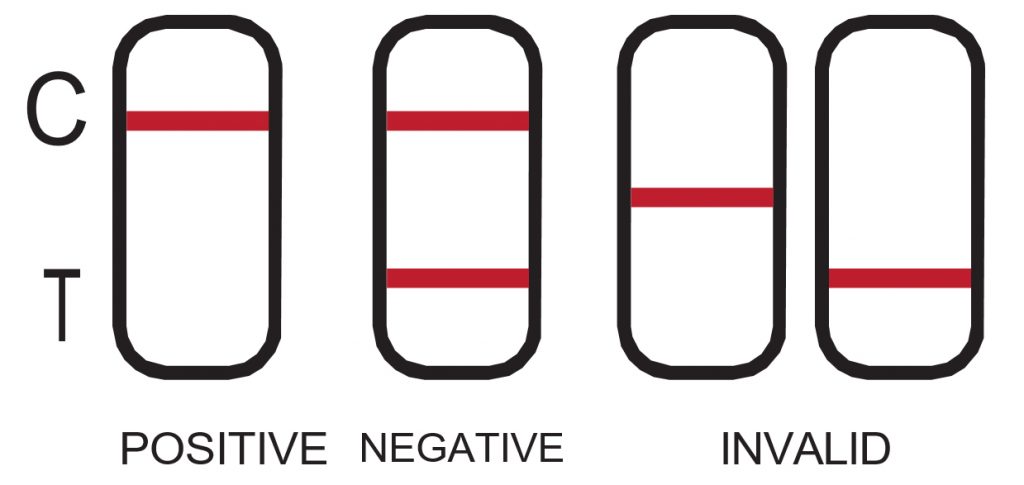
POSITIVE: One red line appears in the control region (C). No line appears in the test region (T). This positive result indicates the drug concentration is above the detectable level. Please refer to the Illustration above.
NEGATIVE: Two lines appear. One red line should be in the control region (C), and another red or weak pink line adjacent appears in the test region (T). This negative result indicates the drug concentration is below the detectable level.
*NOTE: The shade of red in the test line region (T) may vary, but it should be considered negative whenever there is even a faint pink line. Please refer to the Illustration above.
INVALID: Control line fails to appear. Incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test device. If the problem persists, discontinue using the lot immediately and contact your local distributor. Please refer to the Illustration above. The results can be read for a period of up to 10 minutes. DO NOT INTERPRET RESULTS AFTER 10 MINUTES as they will no longer be valid.
Test Results in the Laboratory









Intended Use
The TamperLoks Drug Testing Device is a tamperproof lateral
flow chromatographic immunoassay for the detection of drugs
of abuse and drugs of abuse metabolites in urine. All drug test
strips assembled in the TamperLoks Device will be as specified
by the user.
This assay provides only a preliminary analytical test result.A more
specific alternate chemical method must be used in order to obtain
a confirmed analytical result. Gas chromatography/mass
spectrometry (GC/MS) is the preferred confirmatory method.
Clinical consideration and professional judgment should be applied
to any drug test result, particularly when preliminary positive results
are indicated.
The device is for professional use.

Principle
The TamperLoks Drug Testing Device is an immunoassay based on the principle of competitive binding. Drugs of abuse which may be present in the urine specimen compete against the drug conjugate for binding sites on the antibody. During testing, the urine specimen migrates upward through capillary action. The drugs, if present in the urine specimen below its cut-off concentration, will not saturate the binding sites of its specific antibody. The antibody will then react with the drug-protein conjugate and a visible colored line will appear in the test line above the specific drug test strip. The presence of drugs above the cut-off concentration will saturate all the binding sites of the antibody. Therefore, the colored line will not form in the test line region. To serve as a procedural control, a colored line will always appear at the control line region indicating the proper volume of specimen has been added and membrane wicking has occurred.
Reagents
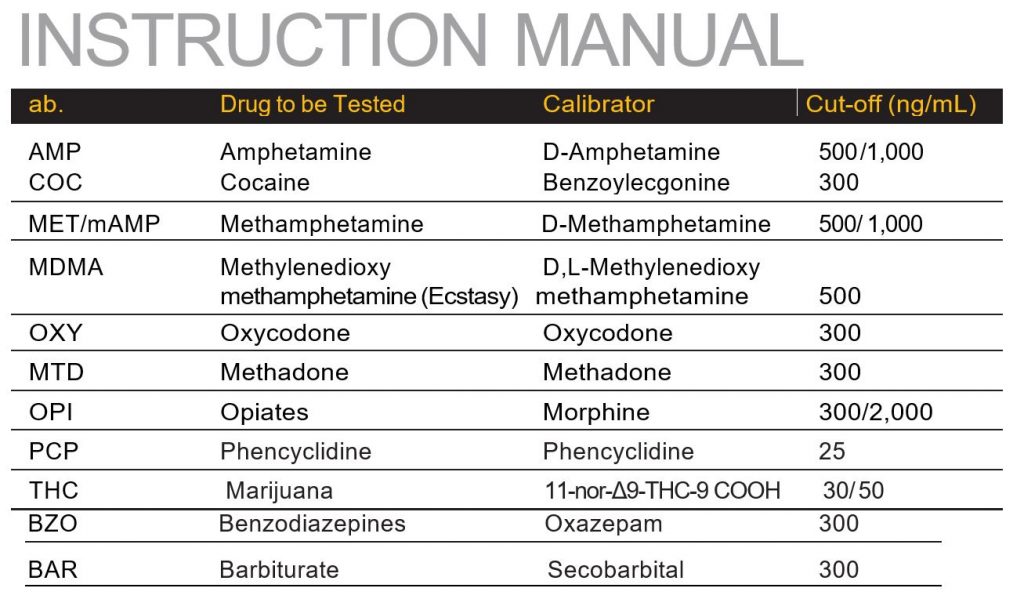
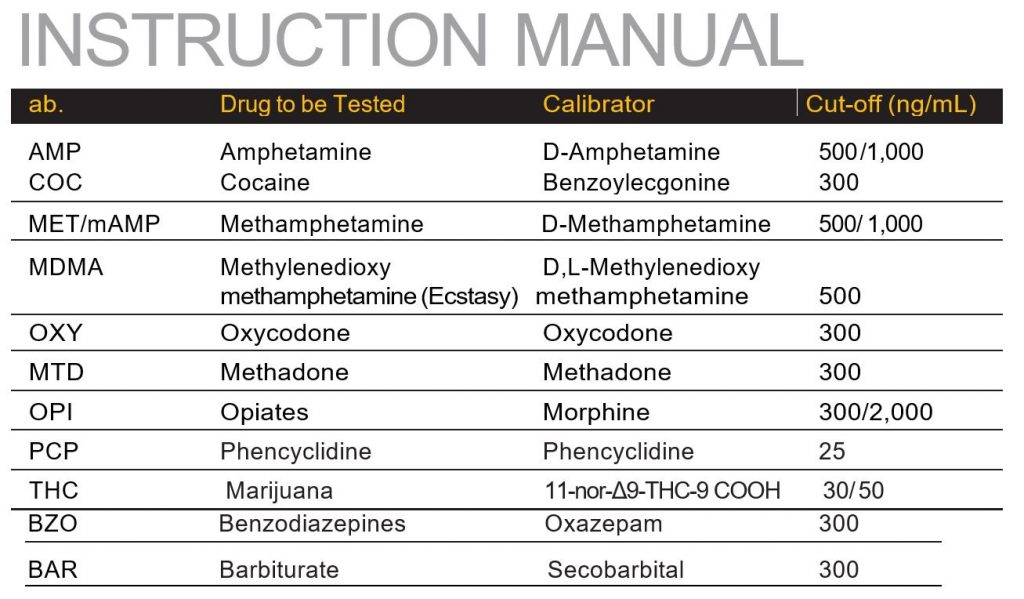
Each test contains a membrane strip coated with drug-protein conjugates (purified bovine albumin) on the test line; a goat polyclonal antibody against gold-protein conjugate at the control line; and a dye pad which contains colloidal gold particles coated with mouse monoclonal antibody specific to Amphetamine, Cocaine, Methamphetamine, Methylenedioxymethamphetamine, Tricyclic Antidepressants, Morphine, THC, Phencyclidine, Benzodiazepine, Methadone or Barbiturate depending on the drug tests specified by the user to be tested.
Storage And Stability
Store as packed in the sealed pouch at 2-30°C/35.6-86°F. The test is stable through the expiration date printed on the sealed pouch. The TamperLoks Test Device must remain in the sealed pouch until use. DO NOT FREEZE. Shelf life: 18 months from date of manufacture.
Precautions
For in vitro diagnostic use only.
Do not use after the expiration date.
All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
The used test device should be discarded according to local regulations.
Collection And Preparation
Urine Assay
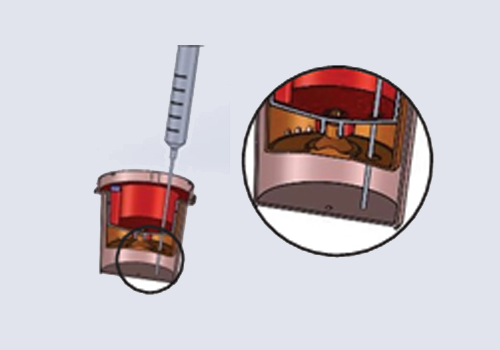
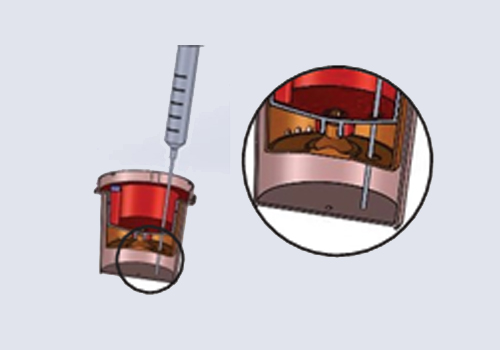
The urine specimen must be collected in a clean and dry TamperLoks Device, specifically in the Upper Collection Chamber. The test can be conducted with urine samples taken at any time of the day. Urine specimens exhibiting visible precipitates should be centrifuged, filtered, or allowed to settle to obtain a clear specimen for testing.
Specimen Storage
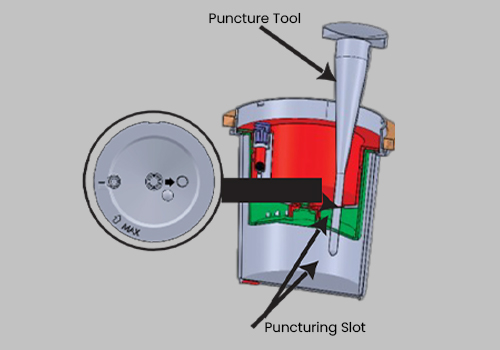
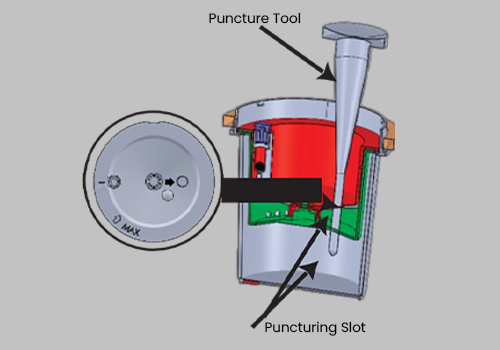
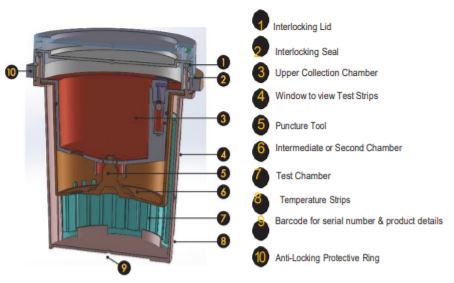
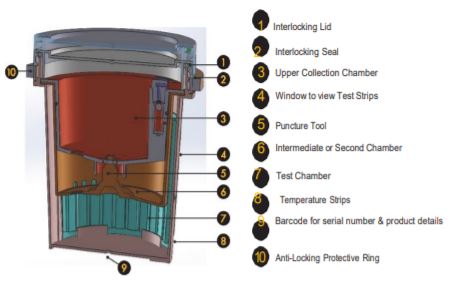
Tamperlok test device



Manually record donorʼs biographical information, as well as the test device serial number.



When ready to begin testing, remove the test device from the sealed pouch.
Do not tear open the pouch until ready to begin testing.



Fill in the requested information on the device (top and side) using a non-erasable ballpoint pen or permanent marker.
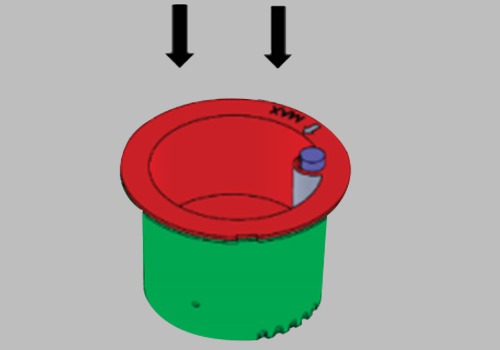

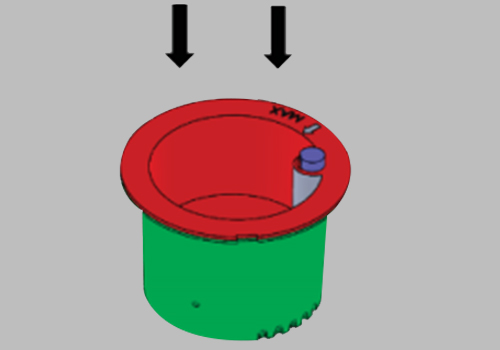
Issue the device to the donor and have them void directly into the Upper Collection Chamber. Ensure the urine specimen is below the maximum fill line.



The device must be returned to you. Place the Interlocking Lid loosely on top, ensuring the Anti-Locking Protective Ring
is not removed from the device at this point.



Check the device to reconfirm the serial number matches the one issued earlier to the donor as recorded in Step 1.




Open the Interlocking Lid and pour off any excess urine above the maximum level indicator. Remove the
Anti-Locking Protective Ring and level the Interlocking Lid to the device evenly.



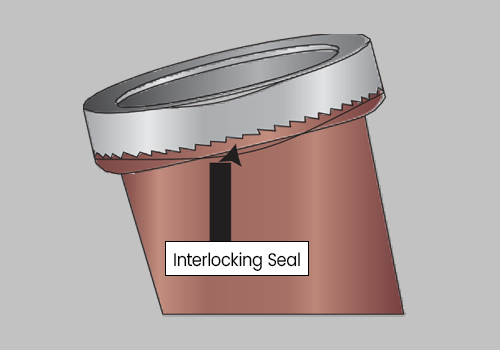
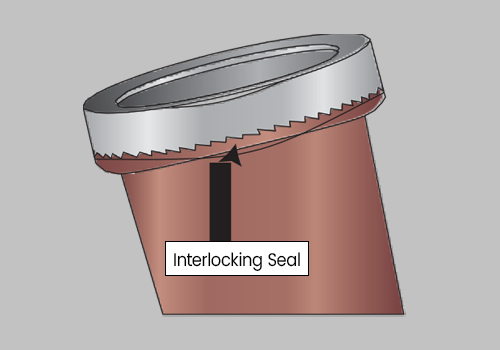
Twist the Lid until tightly secured. Be sure the Interlocking Lid is locked into the Interlocking Seal.
DO NOT ATTEMPT TO REOPEN THE LID, AS THE INTERLOCKING SEAL WILL BREAK.

Allow the urine specimen to flow down to the Test Chamber. Wait FIVE MINUTES to
read/record results from the window showing the test results.
DO NOT INTERPRET RESULTS AFTER 10 MINUTES AS THEY WILL NO LONGER BE VALID.



Also check the Temperature Strip on the deviceʼs outside wall to ensure the sample is fresh/sufficiently thawed. The normal temp range should be 37°C/98.6°F+/-0.61°C/1°F.

If there are any positive results, generate a laboratory confirmation test request. If the positive field test samples are used for confirmatory tests, they must be conducted within the specific storage time and conditions prescribed for the drug being tested.




Twist the Lid until tightly secured. Be sure the Interlocking Lid is locked into the Interlocking Seal.
DO NOT ATTEMPT TO REOPEN THE LID, AS THE INTERLOCKING SEAL WILL BREAK.


































